Platelet Serotonin in Disturbed Monkeys and Children*
By Mary Coleman, M.D.**
From Clinical Proceedings: Children's Hopsital Washington, D.C., Volume 27, July/August 1971, Number 7 (p. 187-194)
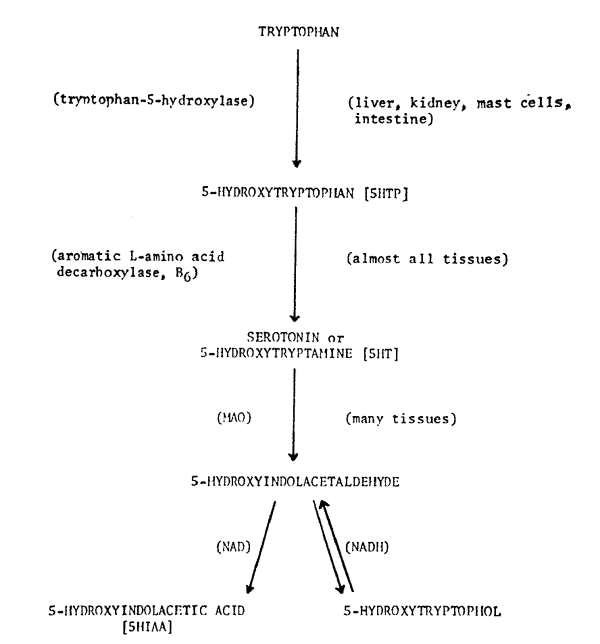
Today we are going to take a look at a biochemical correlate of disturbed behavior. The head banging, rocking and aggressive behavior seen in the maternally deprived animals in the earlier part of this symposium are produced by central nervous system pathways and mediated by their transmitter substances. In the 1950's, the biogenic amine–serotonin–was intensively investigated because there was indirect evidence that it was an amine involved in psychiatric symptoms. In the 1960's, studies on serotonin were overshadowed by papers on the catecholamine pathways, a changeover decreed by scientific fashion. In the 1970's, we suspect that an emphasis will be placed not on serotonin, not on catecholamines, but on the functional balance or functional ratio between them. Most of the enzyme and co-enzyme systems of the serotonin pathway, seen in Figure 1, are shared by its cousin catecholarnine pathway. Also, active transport systems and binding sites are partially shared. Studies of the turnover rates and ratios of bound endogenous levels of these amines to each other may be necessary to explain clinical phenomena. In today's paper, we are reporting only levels of one amine in disturbed monkeys and children. So we are examining only one factor in a complex balance system.
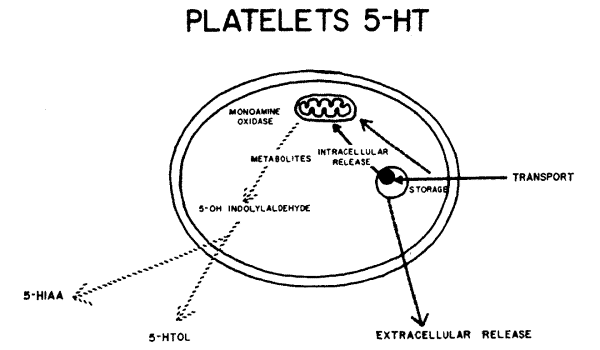
Our work is predominantly with children, rather than with experimental animals. We can't take out and study brain cells containing serotonin; thus we primarily are interested in model systems in other body cells which also happen to contain the brain amines. One such model system is the platelet model of the serotonergic neuron1-3 (Fig. 2). Ninety-nine per cent of all serotonin in blood samples is inside the platelet. This platelet serotonin model has been useful in studies of selected patients with cerebral dysfunctions. However, until an adequate model for the catecholamine
* Presented at the American Psychological Association Meeting, September 1970
** Associate in Neurology, Children's Hospital; Assistant Professor of Neurology, George Washington University School of Medicine. Reprint requests to Children's Hospital, 2125 13th St.9 N. W., Washington, D. C. 20009 (Dr. Coleman). [E.M.: outdated, not removed in order to maintain source authenticity]
p. 188, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
neuron is developed, we are limited to studying one side of that serotonin-catecholamine balanced scale in living animals.
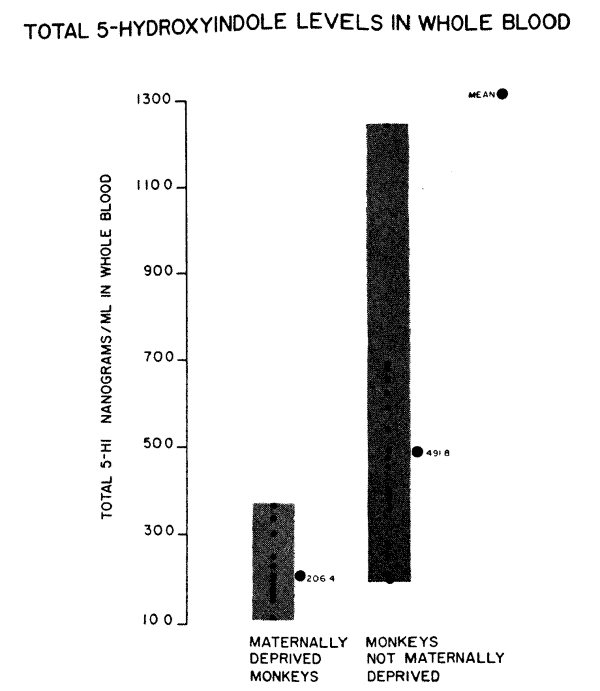
We have studied the serotonin levels in whole blood in 18 rhesus monkeys (obtained through the courtesy of Dr. James Prescott, NIH) who were born in the wilds, lived with their mothers until captured at one year of age, and are presently living in individual wire cages. In addition, we have studied 14 maternally deprived monkeys who were separated from their mothers at birth, hand fed in incubators and have been living in individual wire cages with a minimum of physical handling. As can be seen in Figure 3, there is a significant difference between the two groups. The monkeys born in the wilds (ages 18 months to 2 years), have an arithmetic mean of 492 nanograms/ml of serotonin and the maternally deprived monkeys (ages 8 to 14 months), have an arithmetic mean of 206 nanograms/ml.
Factors of diet and lab error have been compensated for so they would not account for these differences. Could environmental differences, related to types of stress, effect these levels? The blood samples on the
p. 189, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
maternally reared monkeys were not taken in the jungle where these happy, carefree monkeys (or so we imagine) lived the first year of their life. Since then, they have been subjected to the stress of capture, separation and are now living isolated in individual cages. Their reaction to stress could raise serotonin just as easily as the maternally deprived monkeys' reaction to stress could lower it.
Which group of monkeys have normal monkey values? The answer probably is neither group is completely normal and the maternally reared group is reflecting the more acute effects of separation and isolation stress while the maternally deprived group is reflecting a more chronic isolation stress phenomena. This interpretation is based mostly on our previous clinical experience with children with psychiatric symptoms. This interpretation is most extraordinary in the history of science–we are relying on previous human data to interpret monkey data!
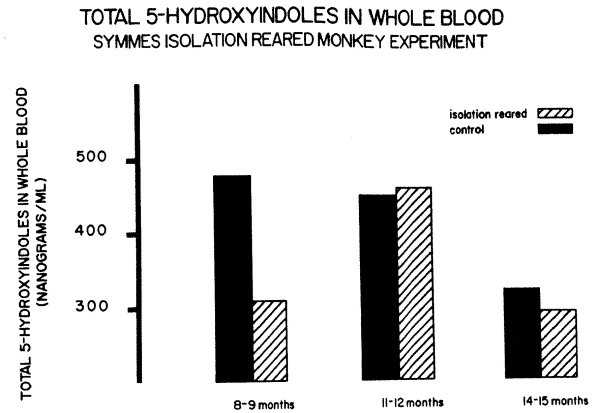
However, there was one additional factor that was not held constant in the Prescott experiment: this was the younger age of the maternally reared monkeys. So another experiment was performed with a group of monkeys raised by David Symmes, also at NIH.4 In this group, both maternally reared and maternally deprived were within one month of age of each other. In Figure 4, it can be seen that the same phenomena of depressed serotonin in maternally deprived monkeys was seen in the first experimental blood sample taken at 8-9 months of age. However, this pattern did not persist when later blood samples were taken. This might be explained either by a tendency of age to compensate for these factors or by the fact that the Symmes experimental animals were all starting to be handled for various experiments.
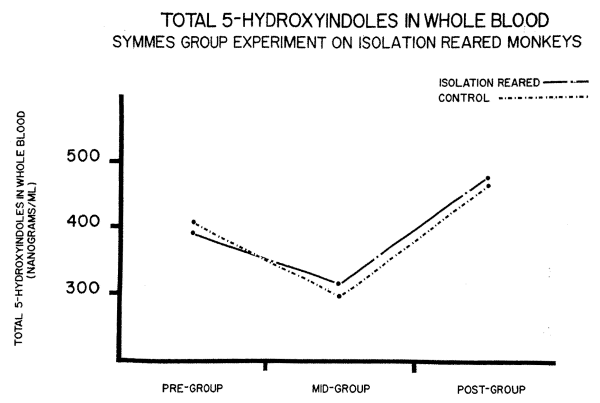
One of the most interesting factors in the Symmes experiment was the seTotonin levels during a grouping experiment (Fig. 5). For a 14 day
p. 190, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
period, each monkey in the two groups was placed with the other members of his group. Although there were marked differences in behavior in the two groups, the serotonin levels of both followed essentially simillar patterns of an initial lowering following a rise above the pre-group baseline. This is reminiscent of the Welch experiments on isolation reared rats which showed "grouping initially lowered brain norepinephrine then led to increased levels."5
How stress can effect serotonin levels also is under investigation in a number of laboratories studying the relationship of adrenocorticosteroids and serotonin. The literature appears contradictory, but the preponderance of evidence suggests that raising the adrenocorticosteroids in acute stress may stimulate the rate-limiting, enzymatic step of serotonin metabolisin (tryptophan hydroxylase) and cause increased turnover of serotonin in the brain; below normal turnover occurs when an animal is adrenalec-
p. 191, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
tomized.6-8 Clinical support of these laboratory studies is paralleled in the Down's syndrome. When these children become ill with fever (raising their adrenocorticosteroids) the dose of 5-HTP has to be temporarily lowered or else symptoms of serotonin overdosage occur.
On the other hand, chronic stress in animals may eventually lower serotonin levels.9 One unlikely explanation is that stimulation of tryptophan pyrrolase in the liver with steroids may eventually shunt the precursor amino acid, tryptophan, to another pathway away from the serotonin pathway.10, 11
In published studies on human beings, there is considerable suggestive but not conclusive literature relating lowered 5-hydroxyindole metabolisin and clinical symptoms of depression.12-21 A recent paper by Bunney and his colleagues raises the possibility of two types of clinical depression–a retarded, slowed-down patient with a catecholamine deficit and an anxious, agitated patient with a serotonin deficit.22
As we were listening earlier to papers describing the symptoms of maternally deprived monkeys during adolescence, we were reminded of some of the hyperactive children we have been studying. They appear to be, anxious, agitated children with serotonin deficits.23 The patients investigated were the subgroup of hyperactive children who have normal EEG's, normal neurological examinations and no other evidence of organic disease.
p. 192, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
(Some of the children were studied courtesy of the hyperactive clinic of Dr. Paul Wender of National Institute of Mental Health.) Most of 25 such patients studied in our laboratory have a depressed serotonin level in the platelets.
The most efficacious treatments for hyperactivity are the amphetamines or the monoamine oxidase inhibitors, that is, antidepressant drugs. This helps support a postulate that some of these hyperactive children may, in fact, be depressed although with paradoxical "manic" type of symptoms.
Thus, these low levels of serotonin may be reflecting environmental factors, although at present, there is not enough evidence for any definite conclusions.
There are other types of more severely disturbed children who may exemplify the opposite side of the nature/nuture controversy. In some patient groups, it could be argued that abnormalities in the biochemistry of the amines (in metabolic pathways, in the active transport system, the binding factors or in transmitter delivery systems) probably was the initiating event with the psychiatric symptoms following. In our laboratory we have studied patients with Kanner's primary infantile autism. This disease process starts at birth in some cases; some children have an adverse reaction to touch noted by the time they are three days of age. Certainly some of these patients have similar behavior to the maternally
p. 193, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
deprived monkeys and this has led some investigators to believe that the disease is environmentally induced. However, the age of initiation is so early in some cases and the symptomatology so severe that an organic etiology seems likely. As infants, most of the children are described as "quite stiff" and failing to mold to the parent; this is suggestive of high levels of serotonin in the newborn period. High serotonin levels in older patients who were not all primary autistics have been described by Schain and Freedman24, we have seen both abnormally high and abnormally low levels in the 5 to 12 year old primary autistic patients studied in our laboratory.
In work done with Dr. David Boullin and Dr. Robert O'Brien in our Children's Hospital Laboratory and in the National Institute of Mental Health Laboratories, an increased amount of 5-HT efflux from the platelets of patients with primary infantile autism has been demonstrated.25 The results suggest there is a defect in the site inside the cell where serotonin is bound and held for future use. Which factor alters binding or whether this is present from birth is not known at this time.
With the demonstration of biogenic amine abnormalities (whatever their etiology), a new amino acid approach to psychiatric symptoms becomes possible. With 5-HTP we can raise serotonin levels, with L-DOPA the brain levels of the catecholamines. These therapies are relatively straight-forward and affect one amine primarily. They may lead to careful manipulation of the sites of the serotonin/catecholamine balance in the brain in the areas such as the reticular formation of the brain stem, the limbic system and basal ganglia.
If we can alter systems that control muscular tonus,3 then we ought to be able to change systems that control the emotional state by similar titration of amino acid levels.
Can we alter them wisely?
For the future is beginning, both for monkeys and for man.
We are most grateful to the Hazelton Laboratories of Falls Church, Virginia, for the gift of the infant monkeys used in this study. The technical assistance of Jovita Lee, Dolores Guansing, Mohini Kaushik, M.D., Dersh Mahanand, and John F. Petersen, DY.M. was invaluable.
- PAASONEN MK: Platelet 5-hydroxytryptamine as a model in pharmacology. Amer Med Exp Biol Fenn 46: 416, 1968
- PLETSCHER A: Metabolism, transfer and storage of 5-bydroxytryptamine in blood platelets. Brit J Pharmacol 32: 1, 1968
- BAZELON M, PAINE RS, COWIE VA, HUNT P, HOUCK JC, MAHANAND D: Reversal of hypotortia in infants with Down's syndrome by administration of 5-hydroxytryptophan. Lancet 1: 1130, 1967
p. 194, July/August 1971 CLINICAL PROCEEDINGS OF CHILDREN'S HOSPITAL
- SYMMES D, EISENGART MA, HEALY MH: Neurobebavioral studies of isolation reared monkeys. Trans Amer Psychol Ass, to be published.
- WELCH AM: Isolation induced changes in brain blogenic amines. Amer Zool 5: 633, 1965
- AZMITIA EC, JR, ALGERI S, COSTA E: In vivo conversion of 3-H-L-tryptophan into 3H-serotonin in brain areas of adrenalectomized rats. Science 169: 201, 1970
- HANIG JP, AIELLO EL, SEIFTER J: The effects of stress and intravenous 0.9 % NaCl injection on concentrations of whole brain 5-hydroxytryptamine in the neonate chick. J Pharm Pharmacol 22: 317, 1970
- NISTICO G, PREZIOSI P: Brain and liver tryptophan pathways and adrenocortical activation during restraint stress. Pharmacol Res Comm 1: 3,63, 1969
- RIEGE WH, MORIMOTO H: Effects of chronic stress and differential environments upon brain weights and biogenic amine levels in rats. J Comp Physiol Psychol 71: 396, 1970
- ALTMAN K, GREENGARD O: Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest 45: 1527, 1966
- GREEN AR, CURZON G: Decrease of 5-hydroxytryptamine in the brain provoked by hydrocortisone and its prevention by allopurinol. Nature 220: 1095, 1968.
- LAPIN IP, OXENKRUG GF: Intensification of the central serotonergic processes as a possible determinant of the thymoleptic effect. Lancet 1: 132, 1969
- SHAW DM, CAMPS FE, ECCELSTON E: 5-hydroxyptamine in the hindbrain of depressive suicides. Brit J Psychiat 133: 1407, 1967
- BOURNE HR, BUNNEY WE, COLBURN RW, DAVIS JM, DAVIS JN, SHAW DM, COPPEN AJ: Noradrenalin, 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet 2: 805, 1968
- PARE CMB, YEUNG DPH, PRICE K, STACEY RS: 5-hydroxytryptamine, noradrenalin and dopamine in the brainstem, hypothalamus, and caudate nucleus of controls and of patients committing suicide by coal gas poisoning. Lancet 2: 133, 1969
- ASHCROFT GW, CRAWFORD TBB, ECCELSTON E, SHARMAN DF: 5-hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological disease. Lancet 2: 1049, 1966
- DENCKER SJ, MALM M, ROOS BE, WERDINIUS B: Acid monamine metabolism of cerebrospinal fluid in mental depression and mania. J Neurochem 13: 1545, 1966
- RODNIGHT R: Body fluid indoles in mental illness. Int Rev Neurobiol 3: 251, 1961
- PARE CMB, SANDLER M: A clinical and biochemical study of a trial of iproniazid in treatment of depression. J Neurol Neurosurg Psychiat 22: 247, 1959
- BUENO JR, HIMWICH HE: A dualistic approach to some biochemical problems in endogenous depressions. Psychosomatics 8: 82, 1967
- STRÖM-OLSEN R, WEIL-MALHERBE H: Humoral changes in manic depressive psychosis with particular reference to excretion of catecholamines in urine. J Ment Sci 104: 696,1958
- BUNNEY WE, JANOWSKY DS, GOODWIN FK, DAVIS JM, BRODIE HKH, MURPHY DL, CHASE TN: Effect of L-DOPA on depression. Lancet 1: 885, 1969
- COLEMAN M: Serotonin concentrations in whole blood of hyperactive children. J Pediat 6: 985, 1971
- SCHAIN RJ, FREEDMAN DX: Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediat 58: 315, 1961
- BOULLIN DJ, COLEMAN M, O'BRIAN RA: Abnormalities in platelet 5-hydroxytryptamine efflux in patients with infantile autism. Nature 227: 371, 1970